Volume 22, Issue 1 (3-2025)
J Res Dev Nurs Midw 2025, 22(1): 13-18 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mirhosseini S, Abbasi A, Divani A, Askarinezhad F, Hasanpournamaghi S, Grimwood S, et al . The Persian version of caregiver self-efficacy scale (CSES): A psychometric evaluation among family caregivers of patients with cancer. J Res Dev Nurs Midw 2025; 22 (1) :13-18
URL: http://nmj.goums.ac.ir/article-1-1899-en.html
URL: http://nmj.goums.ac.ir/article-1-1899-en.html
Seyedmohammad Mirhosseini1 

 , Ali Abbasi2
, Ali Abbasi2 

 , Anahita Divani3
, Anahita Divani3 

 , Fateme Askarinezhad4
, Fateme Askarinezhad4 

 , Soheila Hasanpournamaghi4
, Soheila Hasanpournamaghi4 

 , Samuel Grimwood5
, Samuel Grimwood5 

 , Ali Montazeri6
, Ali Montazeri6 

 , Hossein Ebrahimi7
, Hossein Ebrahimi7 




 , Ali Abbasi2
, Ali Abbasi2 

 , Anahita Divani3
, Anahita Divani3 

 , Fateme Askarinezhad4
, Fateme Askarinezhad4 

 , Soheila Hasanpournamaghi4
, Soheila Hasanpournamaghi4 

 , Samuel Grimwood5
, Samuel Grimwood5 

 , Ali Montazeri6
, Ali Montazeri6 

 , Hossein Ebrahimi7
, Hossein Ebrahimi7 


1- Department of Nursing, School of Nursing and Midwifery, Shahroud University of Medical Sciences, Shahroud, Iran ; Student Research Committee, School of Nursing and Midwifery, Guilan University of Medical Sciences, Rasht, Iran
2- Department of Nursing, School of Nursing and Midwifery, Shahroud University of Medical Sciences, Shahroud, Iran
3- Department of Medical Surgical Nursing, School of Nursing and Midwifery, Tehran University of Medical Sciences, Tehran, Iran
4- Student Research Committee, School of Nursing and Midwifery, Shahroud University of Medical Sciences, Shahroud, Iran
5- Human Sciences Research Centre, The University of Derby, Derby, United Kingdom
6- Imam Hossein Hospital, Shahroud University of Medical Sciences, Shahroud, Iran
7- Center for Health Related Social and Behavioral Sciences Research, Shahroud University of Medical Sciences, Shahroud, Iran ,ebrahimi@shmu.ac.ir
2- Department of Nursing, School of Nursing and Midwifery, Shahroud University of Medical Sciences, Shahroud, Iran
3- Department of Medical Surgical Nursing, School of Nursing and Midwifery, Tehran University of Medical Sciences, Tehran, Iran
4- Student Research Committee, School of Nursing and Midwifery, Shahroud University of Medical Sciences, Shahroud, Iran
5- Human Sciences Research Centre, The University of Derby, Derby, United Kingdom
6- Imam Hossein Hospital, Shahroud University of Medical Sciences, Shahroud, Iran
7- Center for Health Related Social and Behavioral Sciences Research, Shahroud University of Medical Sciences, Shahroud, Iran ,
Full-Text [PDF 504 kb]
(727 Downloads)
| Abstract (HTML) (2064 Views)
Discussion
In this study, EFA identified two distinct factors in the Persian version of the CSES: patient challenges and caregiver self-management, accounting for 43.9% of the total variance. The original CSES was an 8-item scale with a single-factor structure determined through Principal Components Analysis (PCA) (20). In the Persian version, the factor of patient challenges includes four items reflecting caregivers’ confidence in managing patient-related difficulties. This aligns with previous studies, such as Zhang et al. (2013) validation of a self-efficacy questionnaire for dementia caregivers in China (38) and Serpentini et al. (2021) validation of the Italian Caregiver Inventory (CGI-I) in oncology (39). Kazanowski (2005) similarly highlighted caregiver self-efficacy in handling medication, symptom control and treatment adherence (40). Effective caregiving demands high self-efficacy due to the complexities of cancer care, including disease progression, symptom management, and financial strain (15,41,42). This aligns with Bandura’s social cognitive theory, which emphasizes self-efficacy in coping strategies (43).
The caregiver self-management factor, comprising three items focuses on caregivers’ abilities to seek support and maintain their physical and mental well-being. Similar dimensions have been reported in studies on caregiving self-efficacy, including Zhang et al.'s research (38,44) and Serpentini et al.'s study (2021) “caring for oneself” factor (39). Maintaining well-being is essential for effective caregiving, yet many caregivers neglect their own health, leading to role reversal and emotional exhaustion (9,45). Poor self-management is linked to increased psychological distress and patient behavioral issues, whereas strong self-efficacy enhances mood and quality of life (46,47). Previous validations of similar scales did not employ EFA (39,48).
Confirmatory factor analysis demonstrated a strong model fit, reinforcing the scale’s validity. While the original CSES and Zhang et al.’s (2013) tool lacked CFA validation (20,38), our findings align with De Maria et al.’s (2021) assessment of the Caregiver Self-Efficacy in Contributing to Patient Self-Care Scale in Italy (46) and Suwanno et al.’s (2023) validation of Thai CSES (49). These results support the robustness of our model (39,48).
Discriminant validity was confirmed, with CSES items demonstrating distinct measurement properties. Previous research has linked the original CSES to moderate-to-negative correlations with caregiver burden and depression (20). Serpentini et al. (2021) similarly reported inverse relationships between anxiety, depression, and caregiving scores in the Italian version of the caregiver inventory (39). Other studies have validated similar scales outside oncology through prior methodologies that differed from our approach, which employed the HTMT technique (18,48,50).
Reliability testing showed strong internal consistency, with Cronbach's alpha and McDonald's omega coefficients confirming the scale’s robustness. Ritter et al. (2022) reported high internal consistency (Cronbach's alpha coefficients exceeding 0.8) across different CSES versions, aligning with our results (20). Likewise, Zhang et al.’s (2013) Self-Efficacy Questionnaire for Chinese Family Caregivers (SEQCFC) demonstrated good internal consistency across subscales (38). Previous research has predominantly relied on Cronbach's alpha, consistent without methodology (18,39,46,48,51). McDonald's omega coefficient, which remains stable across varying sample sizes, further validated the scale’s reliability (36). Suwanno et al. (2023) reported a comparable omega coefficient (0.87) for the Thai adaptation of the CSES (49).
Test-retest analysis over two weeks confirmed the scale’s stability, with significant correlations between the initial and follow-up scores. The original CSES reported stability at 0.73, which is satisfactory (20). Similarly, Zhang et al. (2013) found ICC values ranging from 0.64 to 0.85 for SEQCFC, supporting its reliability over time (38). Steffen et al. (2002) also reported ICC values above 0.7 for all three factors in their caregiving self-efficacy scale (18). Greenhawt et al. (2018) further validated a similar caregiver self-efficacy measure, reinforcing the findings of this study (50).
The Persian CSES excludes item number 7 from the original version, resulting in a 7-item scale. Scores range from 1 to 10, with higher scores indicating greater caregiver self-efficacy. The two-factor structure comprises patient challenges (Four items) and caregiver self-management (Three items).
This study was conducted among Iranian cancer caregivers, which limits the generalizability of the findings. Most participants were housewives or self-employed; therefore, future studies should include a broader range of occupations. The family structure in Iranian culture is nuclear and influenced by distinct customs and traditions prevalent in West Asia and the Middle East. As such, it is important to psychometrically validate this scale in other cultural contexts. Additionally, as a self-reported measure, the scale may be susceptible to response bias (52).
Conclusion
The findings of this study demonstrate that the CSES, comprising two factors and seven items, is a valid tool for assessing self-efficacy among family caregivers of Iranian cancer patients. The scale shows strong stability, internal consistency, and construct validity, confirming its effectiveness in measuring caregiver self-efficacy. Furthermore, the CSES proves to be a practical tool in oncology nursing, helping to address the challenges faced by family caregivers of cancer patients.
Acknowledgement
This study was the result of a research project approved by the research deputy of Shahroud University of Medical Sciences under referral code 14010050. We would like to express our gratitude to the caregivers for their invaluable contributions to this study. Additionally, we extend our appreciation to the research deputy of Shahroud University of Medical Sciences for their support throughout the research process.
Funding sources
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical statement
The Ethics Council in Biomedical Research at Shahroud University of Medical Sciences approved this study (IR.SHMU.REC.1401.164). At the start of the research, participants were informed of the study's goals and conditions for participation. The authors followed the Committee on Publication Ethics (COPE) guidelines in publishing their findings. Informed consent was obtained from all participants. The right to withdraw from the study and the privacy and confidentiality of participants' personal information were fully respected. All methods were carried out by relevant guidelines and regulations, or the Declaration of Helsinki.
Conflicts of interest
The authors declare that they have no competing interests.
Author contributions
Study design: SM, AA, AD, HE; Data collection: AD, FA, SH; Data analysis: SM; Study supervision: SM; Manuscript writing: All authors (SM, AA, AD, FA, SH, SG, AM, HE). All authors have read and approved the final manuscript.
Full-Text: (428 Views)
Introduction
Cancer imposes physical, psychological, social and emotional burdens on both patients and their caregivers (1,2). Frequent hospital visits, follow-ups, and long-term home care increase caregiving responsibilities, often leading to stress, depression, and a decline in quality of life (3-5). As cancer progresses, caregivers experience a loss of control, impacting relationships, well-being, and caregiving capacity (6,7). High stress, burnout, and emotional distress, including fear of a losing a loved one, further exacerbate their burden and negatively affect patient outcomes (8,9). Therefore, caregivers need support to manage the challenges of their role (10). Despite these challenges, caregiving can also be rewarding, fostering skill development, personal growth and resilience (11). Caregivers who view their role positively often experience greater satisfaction and improved communication (12). Caregiver self-efficacy (The belief in one’s ability to manage caregiving tasks) is a key factor in mitigating stress and enhancing well-being (13). Higher caregiver self-efficacy is associated with lower stress, anxiety and better self-care, while lower self-efficacy correlates with increased psychological distress, such as anxiety and depression, especially assisting spouses that are in considerable pain and other cancer symptoms (14-16). Various psychometric inventories, such as the Caregiver Inventory and the Caring Self-Efficacy Scale, assess caregiver self-efficacy, which includes caregivers of elderly individuals with cognitive issues (17-19). However, there is a lack of accessible caregiver inventories for Iranian caregivers of patients with cancer and for nurses to use in clinical practice and for research studies. This study addresses this gap by examining the psychometric properties of the Caregiver Self-Efficacy Scale (CSES-8), which has not yet undergone psychometric testing or been translated into other cultural contexts, and is therefore not applicable to Iranian caregivers (20).
Methods
Study design
The present methodological study was assessed the psychometric properties of the Persian version the CSES-8 among family caregivers of cancer patients at Imam Hossein Hospital, Shahroud, Iran, from January to May 2024.
Participants and the study setting
According to Munro's recommendations, it is advised to involve between five and ten family caregivers of cancer patients per scale item for both exploratory factor analysis (EFA) and confirmatory factor analysis (CFA) (21). For more precise analyses in this study, 382 family caregivers were evaluated based on specific inclusion criteria. These criteria required participants to be at least 18 years old, have provided care for a minimum of six months (22), be literate in reading and writing, proficient in Persian, not using neuroleptic drugs, and have a confirmed cancer diagnosis by an oncologist. The participants were excluded if they had a diagnosed psychiatric disorder that significantly impaired daily functioning, such as schizophrenia, bipolar disorder, or major depressive disorder with psychotic features. The exclusion criteria included those who have either passed away or been transferred to other medical facilities, making their caregivers unavailable for assessment.
Scale
The 8-item CSES-8 was developed by Ritter et al. (2020). In the previous study, the findings demonstrated that the Principal Component Analysis (PCA) identified a single factor. The CSES-8 initially showed a moderate negative correlation with the Zarit Burden Inventory (ZBI) (r = -0.66, p <0.001), the Patient Health Questionnaire (r = -0.53, p <0.001), and the Caregiver Strain Index (CSI) (r = -0.46, p <0.001), with the strongest correlation observed with the ZBI and the weakest with the CSI. Internal consistency and stability were assessed using Cronbach's alpha (0.88) and ICCs (0.75), respectively. The original version of CSES comprises 8 items rated on a 10-point scale ranging from "Not at all confident" = 1 to "Totally" = 10. A higher score on each item reflects greater caregiver self-efficacy, with caregivers assigning numerical values based on their perceived confidence. The single-factor scale's score is derived as the average of these eight items, yielding a total scale score ranging from 1 to 10 (20).
Translation
Following an email request to Dr. Philip L. Ritter, permission for translation was granted on May 1, 2022. Upon receiving authorization to translate the scale, it underwent translation into Persian following the guidelines outlined by Gudmundsson using a forward-backward approach (23). Two English-fluent translators initially translated the CSES into Persian. Subsequently, the Persian translations were independently translated back into English by another two translators, and the definitive Persian version of the scale was chosen based on recommendations from the research team members.
Face validity
To assess the clarity and relevance of the items within the structure, face validity was employed using both quantitative and qualitative methods. The Impact score (Impact score = Frequency (%) * Importance) was utilized to evaluate how well items aligned with the construct. This score was computed based on a 5-point Likert scale ranging from 1 (Not important) to 5 (Very important), where Frequency indicates the number of respondents selecting options 4 and 5, and Importance represents the average score per item. Items scoring above the cutoff of 1.5 were deemed to have acceptable face validity. Items scoring below this threshold were not discarded but rather revised (22). To obtain feedback for this validity type, ten family caregivers of cancer patients provided insights on appropriateness, difficulty, relevance, and clarity through face-to-face interviews (24).
Content validity
Content validity assesses how well the items in an instrument measure the intended concept for a specific assessment. It was evaluated using both quantitative and qualitative methods. Twelve experts from diverse backgrounds (Including professional nurses, psychologists, and psychometricians) were consulted to assess the clarity of language, appropriateness of terminology, and sequence of items (25). Quantitatively, content validity was evaluated using two measures: Content Validity Ratio (CVR) and Content Validity Index (CVI), with a focus on relevance. Each expert rated the necessity of each item on a three-point scale (1 = necessary, 2 = useful but not necessary, 3 = unnecessary). CVR was calculated using the formula CVR = (ne - [N / 2]) / (N / 2), where ‘ne’ is the number of experts rating the item as "essential," and N is the total number of experts. According to Lawshe's table, for a panel of 12 experts, a CVR score higher than 0.56 indicates content validity (26).
On the other hand, CVI assesses the extent to which the items of the scale are straightforward, relevant, and clear. The same panel of 12 experts rated the simplicity, relevance, and clarity of the CSES items using a four-point scale ranging from 1 to 4. The scale included options such as "not relevant," "somewhat relevant," "completely relevant," and "very relevant," scored as 1, 2, 3, and 4, respectively. Each item's CVI was calculated by dividing the number of experts who rated it as 3 or 4 by the total number of experts. A CVI score above 0.79 indicated adequate content validity, scores between 0.79 and 0.70 were considered questionable, and scores below 0.70 were deemed unacceptable (27). Additionally, the modified Kappa statistics were computed for each item to account for chance agreement among the expert group, with items having a Kappa value of 0.7 or higher considered appropriate.
Construct validity
Construct validity was assessed through EFA and CFA. EFA utilized the maximum likelihood (ML) method with promax rotation on a sample of 191 family caregivers. To provide a more accurate estimation of explained variance, component extraction was conducted using the ML method (28). Data adequacy for EFA was determined using Kaiser-Meyer-Olkin (KMO) criteria and Bartlett's test of sphericity. KMO values falling between 0.6 and 0.7 were deemed acceptable, 0.7 to 0.8 as good, and 0.8 to 0.9 as excellent (29,30). Factor loadings of approximately 0.33 were used to ascertain an item's presence within a latent factor, estimated by the formula: CV = 5.152 ÷ √ (n - 2), where CV represented the number of extractable factors and n was the sample size. Items with a commonality below 0.3 were removed in subsequent EFA steps to adhere to the rule of having at least three items per factor (31). Following EFA, the model's goodness of fit was verified using CFA. This step aimed to confirm that the model derived from EFA accurately represented the true structure within the study population, ensuring a good fit. Various fit indices were employed: Comparative Fit Index (CFI), Normed Fit Index (NFI), Goodness of Fit Index (GFI), Adjusted Goodness of Fit Index (AGFI), Relative Fit Index (RFI), Incremental Fit Index (IFI), Parsimonious Comparative Fit Index (PCFI), and Parsimonious Normed Fit Index (PNFI). Indices exceeding 0.9 were considered satisfactory. Additionally, a Root Mean Square Error of Approximation (RMSEA) value below 0.08 indicated a good fit (32).
Discriminant validity
For assessing discriminant validity, this study employed the HTMT, where each HTMT ratio between constructs should be below 0.85 to confirm discriminant validity. The HTMT ratio compares correlations between different constructs as a measure of discriminant validity (33). Higher HTMT ratios suggest inadequate discriminant validity between constructs (34).
Reliability
Next, two approaches, stability and internal consistency, were employed to assess the reliability of CSES. Stability was assessed using the test-retest method by calculating the intraclass correlation coefficient (ICC), with a minimum acceptable threshold of 0.75, based on the participation of 30 caregivers. Internal consistency was assessed using Cronbach's alpha and McDonald's omega coefficient. Values above 0.7 for both alpha and omega indicate strong internal consistency (35). Additionally, construct reliability (CR) was examined for each factor, with values exceeding 0.7 considered indicative of reliable constructs (36).
Data analysis
Univariate and multivariate outliers were assessed using distribution charts and Mahalanobis distance (Mahalanobis distance p <0.001). Additionally, the normality of univariate distribution (Skewness within ±3 and kurtosis within ±7) and multivariate distribution (Mardia coefficient <8) was examined (37). The data showed no significant departure from normal distribution. For the CFA, missing data were handled using the listwise deletion method due to non-response issues associated with incomplete forms. Statistical analyses were conducted using SPSS and AMOS version 26.0 software.
Results
A total of 382 family caregivers of cancer patients participated in the study. Among them, 177 individuals (46.3%) were women, and 112 (29.3%) were spouses of the patients. The majority, 303 caregivers (79.3%), were married. On average, caregivers spent 7.29 ± 7.65 hours per day caring for their patients. A total of 59 patients (15.4%) had been diagnosed with colon cancer, with an average time since diagnosis of 2.76 ± 2.80 years. Additional details are presented in Table 1.
Cancer imposes physical, psychological, social and emotional burdens on both patients and their caregivers (1,2). Frequent hospital visits, follow-ups, and long-term home care increase caregiving responsibilities, often leading to stress, depression, and a decline in quality of life (3-5). As cancer progresses, caregivers experience a loss of control, impacting relationships, well-being, and caregiving capacity (6,7). High stress, burnout, and emotional distress, including fear of a losing a loved one, further exacerbate their burden and negatively affect patient outcomes (8,9). Therefore, caregivers need support to manage the challenges of their role (10). Despite these challenges, caregiving can also be rewarding, fostering skill development, personal growth and resilience (11). Caregivers who view their role positively often experience greater satisfaction and improved communication (12). Caregiver self-efficacy (The belief in one’s ability to manage caregiving tasks) is a key factor in mitigating stress and enhancing well-being (13). Higher caregiver self-efficacy is associated with lower stress, anxiety and better self-care, while lower self-efficacy correlates with increased psychological distress, such as anxiety and depression, especially assisting spouses that are in considerable pain and other cancer symptoms (14-16). Various psychometric inventories, such as the Caregiver Inventory and the Caring Self-Efficacy Scale, assess caregiver self-efficacy, which includes caregivers of elderly individuals with cognitive issues (17-19). However, there is a lack of accessible caregiver inventories for Iranian caregivers of patients with cancer and for nurses to use in clinical practice and for research studies. This study addresses this gap by examining the psychometric properties of the Caregiver Self-Efficacy Scale (CSES-8), which has not yet undergone psychometric testing or been translated into other cultural contexts, and is therefore not applicable to Iranian caregivers (20).
Methods
Study design
The present methodological study was assessed the psychometric properties of the Persian version the CSES-8 among family caregivers of cancer patients at Imam Hossein Hospital, Shahroud, Iran, from January to May 2024.
Participants and the study setting
According to Munro's recommendations, it is advised to involve between five and ten family caregivers of cancer patients per scale item for both exploratory factor analysis (EFA) and confirmatory factor analysis (CFA) (21). For more precise analyses in this study, 382 family caregivers were evaluated based on specific inclusion criteria. These criteria required participants to be at least 18 years old, have provided care for a minimum of six months (22), be literate in reading and writing, proficient in Persian, not using neuroleptic drugs, and have a confirmed cancer diagnosis by an oncologist. The participants were excluded if they had a diagnosed psychiatric disorder that significantly impaired daily functioning, such as schizophrenia, bipolar disorder, or major depressive disorder with psychotic features. The exclusion criteria included those who have either passed away or been transferred to other medical facilities, making their caregivers unavailable for assessment.
Scale
The 8-item CSES-8 was developed by Ritter et al. (2020). In the previous study, the findings demonstrated that the Principal Component Analysis (PCA) identified a single factor. The CSES-8 initially showed a moderate negative correlation with the Zarit Burden Inventory (ZBI) (r = -0.66, p <0.001), the Patient Health Questionnaire (r = -0.53, p <0.001), and the Caregiver Strain Index (CSI) (r = -0.46, p <0.001), with the strongest correlation observed with the ZBI and the weakest with the CSI. Internal consistency and stability were assessed using Cronbach's alpha (0.88) and ICCs (0.75), respectively. The original version of CSES comprises 8 items rated on a 10-point scale ranging from "Not at all confident" = 1 to "Totally" = 10. A higher score on each item reflects greater caregiver self-efficacy, with caregivers assigning numerical values based on their perceived confidence. The single-factor scale's score is derived as the average of these eight items, yielding a total scale score ranging from 1 to 10 (20).
Translation
Following an email request to Dr. Philip L. Ritter, permission for translation was granted on May 1, 2022. Upon receiving authorization to translate the scale, it underwent translation into Persian following the guidelines outlined by Gudmundsson using a forward-backward approach (23). Two English-fluent translators initially translated the CSES into Persian. Subsequently, the Persian translations were independently translated back into English by another two translators, and the definitive Persian version of the scale was chosen based on recommendations from the research team members.
Face validity
To assess the clarity and relevance of the items within the structure, face validity was employed using both quantitative and qualitative methods. The Impact score (Impact score = Frequency (%) * Importance) was utilized to evaluate how well items aligned with the construct. This score was computed based on a 5-point Likert scale ranging from 1 (Not important) to 5 (Very important), where Frequency indicates the number of respondents selecting options 4 and 5, and Importance represents the average score per item. Items scoring above the cutoff of 1.5 were deemed to have acceptable face validity. Items scoring below this threshold were not discarded but rather revised (22). To obtain feedback for this validity type, ten family caregivers of cancer patients provided insights on appropriateness, difficulty, relevance, and clarity through face-to-face interviews (24).
Content validity
Content validity assesses how well the items in an instrument measure the intended concept for a specific assessment. It was evaluated using both quantitative and qualitative methods. Twelve experts from diverse backgrounds (Including professional nurses, psychologists, and psychometricians) were consulted to assess the clarity of language, appropriateness of terminology, and sequence of items (25). Quantitatively, content validity was evaluated using two measures: Content Validity Ratio (CVR) and Content Validity Index (CVI), with a focus on relevance. Each expert rated the necessity of each item on a three-point scale (1 = necessary, 2 = useful but not necessary, 3 = unnecessary). CVR was calculated using the formula CVR = (ne - [N / 2]) / (N / 2), where ‘ne’ is the number of experts rating the item as "essential," and N is the total number of experts. According to Lawshe's table, for a panel of 12 experts, a CVR score higher than 0.56 indicates content validity (26).
On the other hand, CVI assesses the extent to which the items of the scale are straightforward, relevant, and clear. The same panel of 12 experts rated the simplicity, relevance, and clarity of the CSES items using a four-point scale ranging from 1 to 4. The scale included options such as "not relevant," "somewhat relevant," "completely relevant," and "very relevant," scored as 1, 2, 3, and 4, respectively. Each item's CVI was calculated by dividing the number of experts who rated it as 3 or 4 by the total number of experts. A CVI score above 0.79 indicated adequate content validity, scores between 0.79 and 0.70 were considered questionable, and scores below 0.70 were deemed unacceptable (27). Additionally, the modified Kappa statistics were computed for each item to account for chance agreement among the expert group, with items having a Kappa value of 0.7 or higher considered appropriate.
Construct validity
Construct validity was assessed through EFA and CFA. EFA utilized the maximum likelihood (ML) method with promax rotation on a sample of 191 family caregivers. To provide a more accurate estimation of explained variance, component extraction was conducted using the ML method (28). Data adequacy for EFA was determined using Kaiser-Meyer-Olkin (KMO) criteria and Bartlett's test of sphericity. KMO values falling between 0.6 and 0.7 were deemed acceptable, 0.7 to 0.8 as good, and 0.8 to 0.9 as excellent (29,30). Factor loadings of approximately 0.33 were used to ascertain an item's presence within a latent factor, estimated by the formula: CV = 5.152 ÷ √ (n - 2), where CV represented the number of extractable factors and n was the sample size. Items with a commonality below 0.3 were removed in subsequent EFA steps to adhere to the rule of having at least three items per factor (31). Following EFA, the model's goodness of fit was verified using CFA. This step aimed to confirm that the model derived from EFA accurately represented the true structure within the study population, ensuring a good fit. Various fit indices were employed: Comparative Fit Index (CFI), Normed Fit Index (NFI), Goodness of Fit Index (GFI), Adjusted Goodness of Fit Index (AGFI), Relative Fit Index (RFI), Incremental Fit Index (IFI), Parsimonious Comparative Fit Index (PCFI), and Parsimonious Normed Fit Index (PNFI). Indices exceeding 0.9 were considered satisfactory. Additionally, a Root Mean Square Error of Approximation (RMSEA) value below 0.08 indicated a good fit (32).
Discriminant validity
For assessing discriminant validity, this study employed the HTMT, where each HTMT ratio between constructs should be below 0.85 to confirm discriminant validity. The HTMT ratio compares correlations between different constructs as a measure of discriminant validity (33). Higher HTMT ratios suggest inadequate discriminant validity between constructs (34).
Reliability
Next, two approaches, stability and internal consistency, were employed to assess the reliability of CSES. Stability was assessed using the test-retest method by calculating the intraclass correlation coefficient (ICC), with a minimum acceptable threshold of 0.75, based on the participation of 30 caregivers. Internal consistency was assessed using Cronbach's alpha and McDonald's omega coefficient. Values above 0.7 for both alpha and omega indicate strong internal consistency (35). Additionally, construct reliability (CR) was examined for each factor, with values exceeding 0.7 considered indicative of reliable constructs (36).
Data analysis
Univariate and multivariate outliers were assessed using distribution charts and Mahalanobis distance (Mahalanobis distance p <0.001). Additionally, the normality of univariate distribution (Skewness within ±3 and kurtosis within ±7) and multivariate distribution (Mardia coefficient <8) was examined (37). The data showed no significant departure from normal distribution. For the CFA, missing data were handled using the listwise deletion method due to non-response issues associated with incomplete forms. Statistical analyses were conducted using SPSS and AMOS version 26.0 software.
Results
A total of 382 family caregivers of cancer patients participated in the study. Among them, 177 individuals (46.3%) were women, and 112 (29.3%) were spouses of the patients. The majority, 303 caregivers (79.3%), were married. On average, caregivers spent 7.29 ± 7.65 hours per day caring for their patients. A total of 59 patients (15.4%) had been diagnosed with colon cancer, with an average time since diagnosis of 2.76 ± 2.80 years. Additional details are presented in Table 1.
Table1. Demographic characteristics of cancer patients and caregivers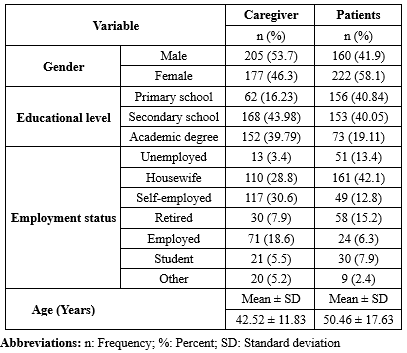 |
The face validity assessment indicated that all scale items were deemed suitable, clear, and significant. Quantitative evaluation of face validity revealed that all scores exceeded 1.5. During qualitative content validity review, adjustments were made to several items based on feedback from 12 experts. Quantitative evaluation using CVR and CVI for each item met the acceptable threshold (0.56), ensuring no items were excluded. Additionally, the modified Kappa statistic indicated strong agreement (Above 0.7) for all items.
In the EFA conducted using the ML method (MLEFA), the KMO measure was 0.644, indicating acceptable sampling adequacy, and Bartlett's test of sphericity yielded a significant result (536.262, p <0.001). Two factors with eigenvalues exceeding one were extracted in this model. As detailed in Table 2, these factors collectively accounted for 43.9% of the total variance. During this phase, item 7 was excluded due to its factor loading being less than 0.3. The results of CFA indicated that all goodness of fit indices supported the final model (χ2 = 37.234, DF = 12, p <0.001, CMIN/DF = 3.103, PCFI = 0.779, PNFI = 0.749, RMSEA = 0.05, IFI = 0.909, GFI = 0.947, AGFI = 0.877, CFI = 0.906, PCFI = 0.518, and PNFI = 0.498).
The HTMT ratio in the current study was assessed at 0.021, confirming discriminant validity among the CSES factors. Cronbach's alpha, McDonald's omega, and the intra-class correlation coefficient (ICC) for the two factors derived from the CSES showed satisfactory results (Table 3). The ICC values for the first and second factors were computed as 0.830 and 0.802, respectively.
In the EFA conducted using the ML method (MLEFA), the KMO measure was 0.644, indicating acceptable sampling adequacy, and Bartlett's test of sphericity yielded a significant result (536.262, p <0.001). Two factors with eigenvalues exceeding one were extracted in this model. As detailed in Table 2, these factors collectively accounted for 43.9% of the total variance. During this phase, item 7 was excluded due to its factor loading being less than 0.3. The results of CFA indicated that all goodness of fit indices supported the final model (χ2 = 37.234, DF = 12, p <0.001, CMIN/DF = 3.103, PCFI = 0.779, PNFI = 0.749, RMSEA = 0.05, IFI = 0.909, GFI = 0.947, AGFI = 0.877, CFI = 0.906, PCFI = 0.518, and PNFI = 0.498).
The HTMT ratio in the current study was assessed at 0.021, confirming discriminant validity among the CSES factors. Cronbach's alpha, McDonald's omega, and the intra-class correlation coefficient (ICC) for the two factors derived from the CSES showed satisfactory results (Table 3). The ICC values for the first and second factors were computed as 0.830 and 0.802, respectively.
Table 2. Exploratory factors analysis of the CSES (N=191)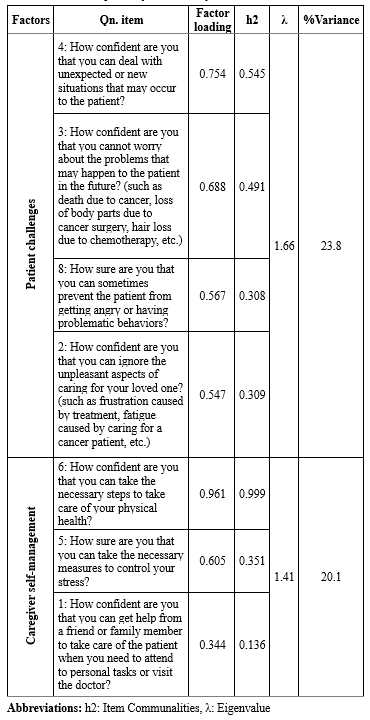 Table 3. Reliability of the CSES .PNG) |
Discussion
In this study, EFA identified two distinct factors in the Persian version of the CSES: patient challenges and caregiver self-management, accounting for 43.9% of the total variance. The original CSES was an 8-item scale with a single-factor structure determined through Principal Components Analysis (PCA) (20). In the Persian version, the factor of patient challenges includes four items reflecting caregivers’ confidence in managing patient-related difficulties. This aligns with previous studies, such as Zhang et al. (2013) validation of a self-efficacy questionnaire for dementia caregivers in China (38) and Serpentini et al. (2021) validation of the Italian Caregiver Inventory (CGI-I) in oncology (39). Kazanowski (2005) similarly highlighted caregiver self-efficacy in handling medication, symptom control and treatment adherence (40). Effective caregiving demands high self-efficacy due to the complexities of cancer care, including disease progression, symptom management, and financial strain (15,41,42). This aligns with Bandura’s social cognitive theory, which emphasizes self-efficacy in coping strategies (43).
The caregiver self-management factor, comprising three items focuses on caregivers’ abilities to seek support and maintain their physical and mental well-being. Similar dimensions have been reported in studies on caregiving self-efficacy, including Zhang et al.'s research (38,44) and Serpentini et al.'s study (2021) “caring for oneself” factor (39). Maintaining well-being is essential for effective caregiving, yet many caregivers neglect their own health, leading to role reversal and emotional exhaustion (9,45). Poor self-management is linked to increased psychological distress and patient behavioral issues, whereas strong self-efficacy enhances mood and quality of life (46,47). Previous validations of similar scales did not employ EFA (39,48).
Confirmatory factor analysis demonstrated a strong model fit, reinforcing the scale’s validity. While the original CSES and Zhang et al.’s (2013) tool lacked CFA validation (20,38), our findings align with De Maria et al.’s (2021) assessment of the Caregiver Self-Efficacy in Contributing to Patient Self-Care Scale in Italy (46) and Suwanno et al.’s (2023) validation of Thai CSES (49). These results support the robustness of our model (39,48).
Discriminant validity was confirmed, with CSES items demonstrating distinct measurement properties. Previous research has linked the original CSES to moderate-to-negative correlations with caregiver burden and depression (20). Serpentini et al. (2021) similarly reported inverse relationships between anxiety, depression, and caregiving scores in the Italian version of the caregiver inventory (39). Other studies have validated similar scales outside oncology through prior methodologies that differed from our approach, which employed the HTMT technique (18,48,50).
Reliability testing showed strong internal consistency, with Cronbach's alpha and McDonald's omega coefficients confirming the scale’s robustness. Ritter et al. (2022) reported high internal consistency (Cronbach's alpha coefficients exceeding 0.8) across different CSES versions, aligning with our results (20). Likewise, Zhang et al.’s (2013) Self-Efficacy Questionnaire for Chinese Family Caregivers (SEQCFC) demonstrated good internal consistency across subscales (38). Previous research has predominantly relied on Cronbach's alpha, consistent without methodology (18,39,46,48,51). McDonald's omega coefficient, which remains stable across varying sample sizes, further validated the scale’s reliability (36). Suwanno et al. (2023) reported a comparable omega coefficient (0.87) for the Thai adaptation of the CSES (49).
Test-retest analysis over two weeks confirmed the scale’s stability, with significant correlations between the initial and follow-up scores. The original CSES reported stability at 0.73, which is satisfactory (20). Similarly, Zhang et al. (2013) found ICC values ranging from 0.64 to 0.85 for SEQCFC, supporting its reliability over time (38). Steffen et al. (2002) also reported ICC values above 0.7 for all three factors in their caregiving self-efficacy scale (18). Greenhawt et al. (2018) further validated a similar caregiver self-efficacy measure, reinforcing the findings of this study (50).
The Persian CSES excludes item number 7 from the original version, resulting in a 7-item scale. Scores range from 1 to 10, with higher scores indicating greater caregiver self-efficacy. The two-factor structure comprises patient challenges (Four items) and caregiver self-management (Three items).
This study was conducted among Iranian cancer caregivers, which limits the generalizability of the findings. Most participants were housewives or self-employed; therefore, future studies should include a broader range of occupations. The family structure in Iranian culture is nuclear and influenced by distinct customs and traditions prevalent in West Asia and the Middle East. As such, it is important to psychometrically validate this scale in other cultural contexts. Additionally, as a self-reported measure, the scale may be susceptible to response bias (52).
Conclusion
The findings of this study demonstrate that the CSES, comprising two factors and seven items, is a valid tool for assessing self-efficacy among family caregivers of Iranian cancer patients. The scale shows strong stability, internal consistency, and construct validity, confirming its effectiveness in measuring caregiver self-efficacy. Furthermore, the CSES proves to be a practical tool in oncology nursing, helping to address the challenges faced by family caregivers of cancer patients.
Acknowledgement
This study was the result of a research project approved by the research deputy of Shahroud University of Medical Sciences under referral code 14010050. We would like to express our gratitude to the caregivers for their invaluable contributions to this study. Additionally, we extend our appreciation to the research deputy of Shahroud University of Medical Sciences for their support throughout the research process.
Funding sources
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical statement
The Ethics Council in Biomedical Research at Shahroud University of Medical Sciences approved this study (IR.SHMU.REC.1401.164). At the start of the research, participants were informed of the study's goals and conditions for participation. The authors followed the Committee on Publication Ethics (COPE) guidelines in publishing their findings. Informed consent was obtained from all participants. The right to withdraw from the study and the privacy and confidentiality of participants' personal information were fully respected. All methods were carried out by relevant guidelines and regulations, or the Declaration of Helsinki.
Conflicts of interest
The authors declare that they have no competing interests.
Author contributions
Study design: SM, AA, AD, HE; Data collection: AD, FA, SH; Data analysis: SM; Study supervision: SM; Manuscript writing: All authors (SM, AA, AD, FA, SH, SG, AM, HE). All authors have read and approved the final manuscript.
Type of study: Original Article |
Subject:
Nursing
References
1. Mirhosseini S, Bazghaleh M, Basirinezhad MH, Abbasi A, Ebrahimi H. Health-related quality of life and caregiver's burden in patients with chronic diseases: a cross-sectional study. Family Medicine & Primary Care Review. 2021;23(1):29-35. [View at Publisher] [DOI] [Google Scholar]
2. Guerra-Martín MD, Casado-Espinosa MDR, Gavira-López Y, Holgado-Castro C, López-Latorre I, Borrallo-Riego Á. Quality of life in caregivers of cancer patients: a literature review. Int J Environ Res Public Health. 2023;20(2):1570. [View at Publisher] [DOI] [PMID] [Google Scholar]
3. Abuatiq A, Brown R, Wolles B, Randall R. Perceptions of stress: patient and caregiver experiences with stressors during hospitalization. Clin J Oncol Nurs. 2020;24(1):51-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
4. Palacio Gonzalez C, Roman‐Calderón JP, Limonero JT. The relationship between positive aspects of caring, anxiety and depression in the caregivers of cancer patients: The mediational role of burden. Eur J Cancer Care (Engl). 2021;30(1):e13346. [View at Publisher] [DOI] [PMID] [Google Scholar]
5. Zhang Y, Zhang S, Liu C, Chen X, Ding Y, Guan C, et al. Caregiver burden among family caregivers of patients with advanced cancer in a palliative context: A mixed‐method study. J Clin Nurs. 2023;32(21-22):7751-64. [View at Publisher] [DOI] [PMID] [Google Scholar]
6. Zhu Y, Pei X, Chen X, Li T. Family caregivers' experiences of caring for advanced cancer patients: A qualitative systematic review and meta-synthesis. Cancer Nurs. 2023;46(4):270-83. [View at Publisher] [DOI] [PMID] [Google Scholar]
7. Paterson C, Roberts C, Li J, Chapman M, Strickland K, Johnston N, et al. What are the experiences of supportive care in people affected by brain cancer and their informal caregivers: a qualitative systematic review. J Cancer Surviv. 2024;18(5):1608-29. [View at Publisher] [DOI] [PMID] [Google Scholar]
8. Bijnsdorp FM, Onwuteaka-Philipsen BD, Boot CR, van der Beek AJ, Pasman HRW. Caregiver's burden at the end of life of their loved one: insights from a longitudinal qualitative study among working family caregivers. BMC Palliat Care. 2022;21(1):142. [View at Publisher] [DOI] [PMID] [Google Scholar]
9. Oliveira C, Lourenço D, Sotero L, Relvas AP. Caregivers' concerns through health professionals' eyes. Palliat Support Care. 2024;22(3):499-510. [View at Publisher] [DOI] [PMID] [Google Scholar]
10. Cai Y, Simons A, Toland S, Zhang J, Zheng K. Informal caregivers' quality of life and management strategies following the transformation of their cancer caregiving role: A qualitative systematic review. Int J Nurs Sci. 2021;8(2):227-36. [View at Publisher] [DOI] [PMID] [Google Scholar]
11. Lee Y, Li L. Evaluating the positive experience of caregiving: A systematic review of the positive aspects of caregiving scale. Gerontologist. 2022;62(9):e493-e507. [View at Publisher] [DOI] [PMID] [Google Scholar]
12. Nemcikova M, Katreniakova Z, Nagyova I. Social support, positive caregiving experience, and caregiver burden in informal caregivers of older adults with dementia. Front Public Health. 2023;11:1104250. [View at Publisher] [DOI] [PMID] [Google Scholar]
13. Lv Q, Zhang X, Wang Y, Xu X, Zang X. Cross-cultural adaptation and validation of the caregiver self-efficacy in contributing to patient self-care scale in China. BMC Public Health. 2024;24(1):1977. [View at Publisher] [DOI] [PMID] [Google Scholar]
14. Phongtankuel V, Moxley J, Reid M, Adelman RD, Czaja SJ. The relationship of caregiver self-efficacy to caregiver outcomes: a correlation and mediation analysis. Aging Ment Health. 2023;27(7):1322-8. [View at Publisher] [DOI] [PMID] [Google Scholar]
15. Hebdon MCT, Coombs LA, Reed P, Crane TE, Badger TA. Self-efficacy in caregivers of adults diagnosed with cancer: An integrative review. Eur J Oncol Nurs. 2021;52:101933. [View at Publisher] [DOI] [PMID] [Google Scholar]
16. Sato H, Nakaaki S, Sato J, Shikimoto R, Furukawa TA, Mimura M, et al. Caregiver self‐efficacy and associated factors among caregivers of patients with dementia with Lewy bodies and caregivers of patients with Alzheimer's disease. Psychogeriatrics. 2021;21(5):783-94. [View at Publisher] [DOI] [PMID] [Google Scholar]
17. Zeiss AM, Gallagher-Thompson D, Lovett S, Rose J, McKibbin C. Self-efficacy as a mediator of caregiver coping: Development and testing of an assessment model. J Clin Neuropsychol. 1999;5(3):221-30. [View at Publisher] [DOI] [Google Scholar]
18. Steffen AM, McKibbin C, Zeiss AM, Gallagher-Thompson D, Bandura A. The revised scale for caregiving self-efficacy: reliability and validity studies. J Gerontol B Psychol Sci Soc Sci. 2002;57(1):P74-86. [View at Publisher] [DOI] [PMID] [Google Scholar]
19. Merluzzi TV, Philip EJ, Vachon DO, Heitzmann CA. Assessment of self-efficacy for caregiving: the critical role of self-care in caregiver stress and burden. Palliat Support Care. 2011;9(1):15-24. [View at Publisher] [DOI] [PMID] [Google Scholar]
20. Ritter P, Sheth K, Stewart A, Gallagher-Thompson D, Lorig K. Development and Evaluation of the 8-item Caregiver Self-Efficacy Scale (CSES-8). The Gerontologist. 2022;62(3):e140-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
21. Munro BH. Statistical methods for health care research. Philadelphia:lippincott williams & wilkins;2005. [View at Publisher] [Google Scholar]
22. Mirhosseini S, Moslemi F, Sharif-Nia H, Minaei-Moghadam S, Khajeh M, Abbasi A, et al. Psychometric evaluation of the Persian version of the Family Caregiver-Specific Quality of Life Scale (FAMQOL) among family caregivers of patients with chronic psychiatric disorders. BMC Psychol. 2025;13(1):222. [View at Publisher] [DOI] [PMID] [Google Scholar]
23. Gudmundsson E. Guidelines for translating and adapting psychological instruments. Nordic Psychology. 2009;61(2):29-45. [View at Publisher] [DOI] [Google Scholar]
24. Mirhosseini S, Ameri F, Rahmani H, Sharif-Nia H, Fazel G, Khajeh M, et al. Psychometric assessment of the persian version of the study anxiety questionnaire in medical sciences students. BMC Med Educ. 2024;24(1):1517. [View at Publisher] [DOI] [PMID] [Google Scholar]
25. Polit-O'Hara D, Hungler BP. Essentials of nursing research: Methods, appraisal, and utilization. Lippincot;1993. [View at Publisher] [Google Scholar]
26. Lawshe CH. A quantitative approach to content validity. Personnel psychology. 1975;28(4):563-75. [View at Publisher] [DOI] [Google Scholar]
27. Lenz ER. Measurement in nursing and health research. New York:Springer publishing company;2010. [View at Publisher] [Google Scholar]
28. Mabel OA, Olayemi OS. A comparison of principal component analysis, maximum likelihood and the principal axis in factor analysis. American Journal of Mathematics and Statistics. 2020;10(2):44-54. [View at Publisher] [Google Scholar]
29. Cattell RB. The scree test for the number of factors.Multivariate Behav Res. 1966;1(2):245-76. [View at Publisher] [DOI] [PMID] [Google Scholar]
30. Feng P, Yang H-L, Xu L, Ojo O, Lu X-Y, Zhang H-Y, et al. Development and psychometric testing of a questionnaire to assess Nurse's perception of risks during enteral nutrition. BMC Nurs. 2021;20(1):6. [View at Publisher] [DOI] [PMID] [Google Scholar]
31. Pett MA, Lackey NR, Sullivan JJ. Making sense of factor analysis: The use of factor analysis for instrument development in health care research. US:sage;2003. [View at Publisher] [DOI] [Google Scholar]
32. Sharif-Nia H, Sivarajan Froelicher E, She L, Jafari-Koulaee A, Hejazi S, Mosazadeh H, et al. The Persian version of the body esteem scale among Iranian adolescents: a translation, psychometrics, and network analysis. Front Psychol. 2024;15:1296498. [View at Publisher] [DOI] [PMID] [Google Scholar]
33. Franke G, Sarstedt M. Heuristics versus statistics in discriminant validity testing: a comparison of four procedures. Internet research. 2019;29(3):430-47. [View at Publisher] [DOI] [Google Scholar]
34. Henseler J, Ringle CM, Sarstedt M. A new criterion for assessing discriminant validity in variance-based structural equation modeling. Journal of the academy of marketing science. 2015;43:115-35. [View at Publisher] [DOI] [Google Scholar]
35. Cho E. Neither Cronbach's alpha nor McDonald's omega: A commentary on Sijtsma and Pfadt. Psychometrika. 2021;86(4):877-86. [View at Publisher] [DOI] [PMID] [Google Scholar]
36. Kalkbrenner MT. Alpha, omega, and H internal consistency reliability estimates: Reviewing these options and when to use them. Counseling Outcome Research and Evaluation. 2023;14(1):77-88. [View at Publisher] [DOI] [Google Scholar]
37. Zygmont C, Smith MR. Robust factor analysis in the presence of normality violations, missing data, and outliers: Empirical questions and possible solutions. The Quantitative Methods for Psychology. 2014;10(1):40-55. [View at Publisher] [DOI] [Google Scholar]
38. Zhang SY, Edwards H, Yates P, Ruth E, Guo Q. Preliminary reliability and validity testing of a Self-Efficacy Questionnaire for Chinese Family Caregivers. Aging Ment Health. 2013;17(5):630-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
39. Serpentini S, Guandalini B, Tosin G, Ronconi L, Cristaldi G, Amatulli R, et al. Assessment of self-efficacy for caregiving in oncology: Italian validation of the caregiver inventory (CGI-I). BMC Palliat Care. 2021;20(1):166. [View at Publisher] [DOI] [PMID] [Google Scholar]
40. Kazanowski M. Family caregivers' medication management of symptoms in patients with cancer near death. J Hosp Palliat Nurs. 2005;7(3):174-81. [View at Publisher] [DOI] [Google Scholar]
41. Lewandowska A, Rudzki G, Lewandowski T, Rudzki S. The problems and needs of patients diagnosed with cancer and their caregivers.Int J Environ Res Public Health. 2020;18(1):87. [View at Publisher] [DOI] [PMID] [Google Scholar]
42. Cheng HL, Leung DYP, Ko PS, Lam WM, Lam PT, Luk AL, et al. Mediating role of self‐efficacy between unmet needs and quality of life in palliative cancer caregivers. Psychooncology. 2023;32(3):457-64. [View at Publisher] [DOI] [PMID] [Google Scholar]
43. Bandura A. Social cognitive theory: An agentic perspective. Annu Rev Psychol. 2001;52(1):1-26. [View at Publisher] [DOI] [PMID] [Google Scholar]
44. Zhang SY, Edwards H, Yates P, Ruth E, Guo QH. Development of self‐efficacy questionnaire for chinese family caregivers. Int J Ment Health Nurs. 2012;21(4):358-65. [View at Publisher] [DOI] [PMID] [Google Scholar]
45. Jun WH, Cha KS, Lee KL. The mediating effect of depression on the relationship between social support, spirituality and burnout in family members of patients with cancer. Int J Environ Res Public Health. 2021;18(4):1727. [View at Publisher] [DOI] [PMID] [Google Scholar]
46. De Maria M, Iovino P, Lorini S, Ausili D, Matarese M, Vellone E. Development and psychometric testing of the caregiver self-efficacy in contributing to patient self-care scale. Value Health. 2021;24(10):1407-15. [View at Publisher] [DOI] [PMID] [Google Scholar]
47. Lee JY, Chang HK. Factors influencing family caregivers' self-management of acute stroke survivors. Korean Journal of Adult Nursing. 2018;30(6):669-80. [View at Publisher] [DOI] [Google Scholar]
48. Hong M, Kim K, Casado BL. Psychometric evaluation of the caregiver self-efficacy scale with Korean Americans. Soc Work Health Care. 2016;55(10):861-73. [View at Publisher] [DOI] [PMID] [Google Scholar]
49. Suwanno J, Klinjun N, Srisomthrong K, Kelly M, Mayeng M, Suwanno J. Validating the caregiver self‐efficacy in contribution to self‐care scale Thai version for stroke: A psychometric evaluation. Nurs Open. 2023;10(11):7360-7. [View at Publisher] [DOI] [PMID] [Google Scholar]
50. Greenhawt M, DunnGalvin A. Preliminary psychometric analyses and clinical performance of a caregiver self-efficacy scale for food allergy self-management. Ann Allergy Asthma Immunol. 2018;120(1):73-9. [View at Publisher] [DOI] [PMID] [Google Scholar]
51. Márquez-González M, Losada A, López J, Peñacoba C. Reliability and validity of the Spanish version of the revised scale for caregiving self-efficacy. Clinical Gerontologist. 2009;32(4):347-57. [View at Publisher] [DOI] [Google Scholar]
52. Giromini L, Young G, Sellbom M. Assessing negative response bias using self-report measures: New articles, new issues. Psychol Inj Law. 2022;15(1):1-21. [View at Publisher] [DOI] [Google Scholar]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



